Answers to selected problems, Chapter 10
Review questions
6. (a) Linear triatomic molecules will (by definition) have bond
angles of 180º. (An example is BeCl2.)
(b) Trigonal planar triatomic molecules will have bond angles
of 120º. (The third outside position will be occupied by an unshared pair of
electrons.) An example is SO2.
(c) A tetrahedral triatomic molecule will have bond angles of
109.5º. (An example is H2O.)
11. Must every molecule have a dipole moment? No. Diatomic elements such as O2 and Cl2 don't, and symmetric heteromolecular molecules such as CCl4 don't, even though their individual bonds are polar.
20. Are all bonds in alkanes σ bonds? Yes, because by definition, alkanes contain only single bonds, which are σ bonds.
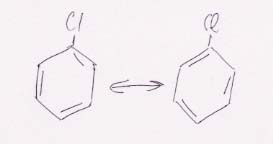
30.

Kekulé structures
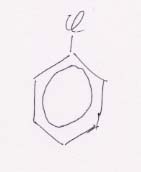
Resonance structure
Problems
31.
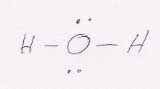
a. AX2E2
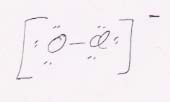
b. AXE3

c. AX2E3
39.

(a) The structure of H2O2 is "bent" by the O–O–H bonds.

(b) The structure of C3O2 is linear because none of the C's have any unshared pairs of electrons.
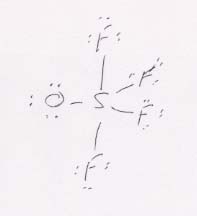
(c) OSF4 has S in the center (because it's the least electronegative of these elements) and the others clustered around it. Overall, it is trigonal bipyramidal, with the O and two of the F's in the central plane. The O is in the central plane because it is larger than the F's, and in this way can be at the greatest possible distance from them.
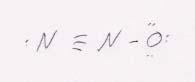
(d) N2O is linear because the central N atom has two bonds and no unshared pairs of electrons.
43. First off, all the bonds in these compounds will be polar to some extent. The molecules will be polar if the bonds are symmetric, nonpolar if they are asymmetric. So you have to determine the structure of each molecule and see it if it symmetric. Here are the structures and their effect on polarity:
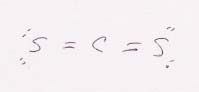
(a) CS2 is linear, symmetric, and therefore nonpolar.

(b) NO2 is bent (because of the lone unshared electron), asymmetric, and therefore polar.
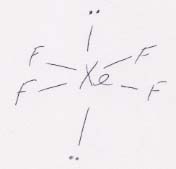
(c) Counting the two unshared pairs of electrons, XeF4 is octahedral. The unshared pairs are at opposite positions in order to get as far away from each other as possible (because the unshared pairs are larger than the electrons in bonds). When only the atoms are considered, though, XeF4 is square planar, symmetric, and therefore nonpolar.
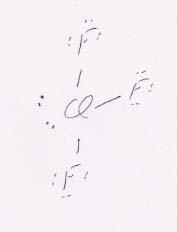
(d) ClF3 is T-shaped (trigonal bipyramidal), asymmetric, and therefore polar.
47.

(a) NF3 is trigonal pyramidal (a pyramid with three feet). It gets that way because it is really a tetrahedral structure with an unshared pair of electrons occupying one of the corners. The three polar bonds, with the electrons more toward the F than the N, make the molecule polar, with the F side being partially negative. This effect is partially canceled by the unshared pair on the other side of the molecule, though. NH3 has the same structure, as shown on page 412.

(b) SF4 is trigonal bipyramidal because it has five "objects," or electron groups, around the S (4 F's and one unshared pair of electrons). The five atoms form a "seesaw" structure. (Figure it out yourself--it really is like a seesaw.) As with (a), the four F atoms make this a polar molecule, but part of the effect is canceled by the lone pair on the central S atom.
49. Li2 is formed by overlapping the 2s orbitals. F2(g), on the other hand, is formed by overlapping the 2px orbitals. F2(g) has the greater bond energy because p orbitals can overlap more than s orbitals can.
55. (a) ClNO2
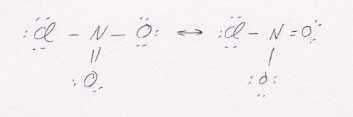
The resonance Lewis structures. The molecular structure is trigonal planar.
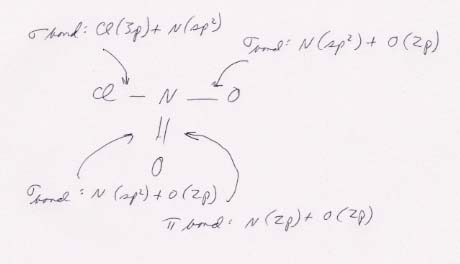
The bonding scheme: the single bonds to N are σ bonds; the double bond between N and O is a σ bond plus a π bond.
(b) OF2

The Lewis structure is straightforward. Its molecular structure is bent. (The F's occupy corners of a tetrahedron.)
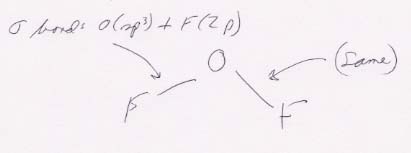
The bonding scheme: 2p orbitals of F overlap with two of the four sp3 hybrid orbitals of O.
(c) CO32-
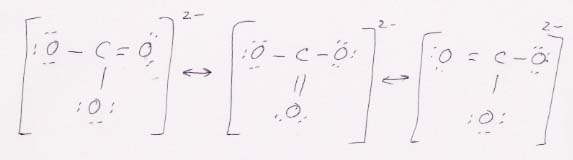
This ion has three resonance Lewis structures. The molecular geometry for each is trigonal planar.

The bonding scheme: 2p orbitals from the O's overlap sp2 orbitals of the C to create the σ bonds; a 2p from the O overlaps a 2p from the C to create the π part of the double bond.
59.
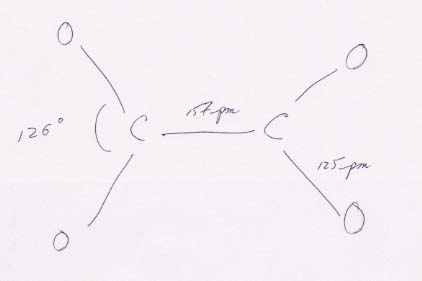
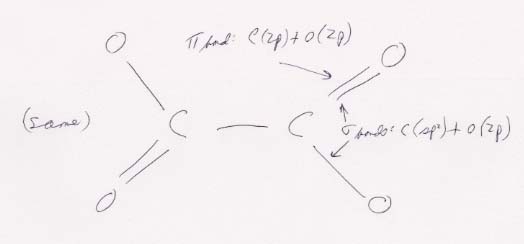
The basic structure of the oxalate ion. The 157 pm for the length of the C–C bond is consistent with a single bond (154 pm; from Table 9.1, on page 391), as it should be. The 125 pm for the four C–O bonds is between the lengths for a single bond (143 pm) and a double bond (120 pm). It is therefore consistent with an intermediate state. The four resonance structures for this configuration are shown below.

The O–C–O angles of 126° are larger than the theoretical 120° because the unshared pairs of electrons on the oxygen atoms at each end of the molecule repel one another and spread the angle. The bonding and hybridization is shown below.
63. Which of the following can exist as cis-trans
isomers? Explain.
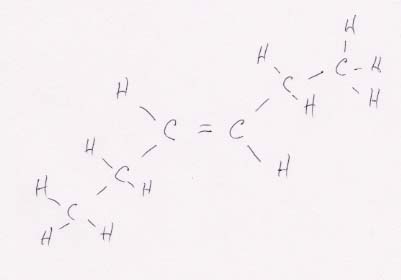
(a) CH3CH2CH==CHCH2CH3
looks like this when it is expanded:
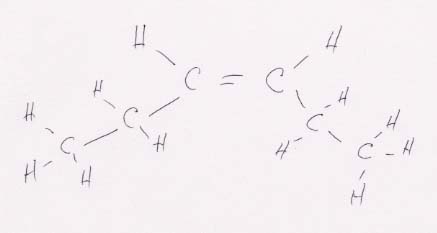
This structure can have cis-trans isomers because both of the central carbons are linked to two different functional groups (H and CH2CH3 in this case). Thus, for example, moving the CH2CH3 group on the right to the top position changes its orientation to the left CH2CH3 group from cis (above) to trans (below).
(b) CH2==CHCH2CH2CH3 looks like this:
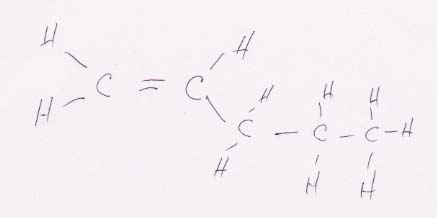
This structure does not have cis-trans isomers because the left carbon is bonded to two identical functional groups (H). That means that each of the functional groups on the right will always be across from a hydrogen no matter whether the right functional group is placed at the top or the bottom. To illustrate this point, see the drawing below, where the right-hand groups have been reversed top to bottom. It remains the same molecule as above, just flipped about the horizontal axis.

(c) CH3CH2CH==CHCH3 looks like this:
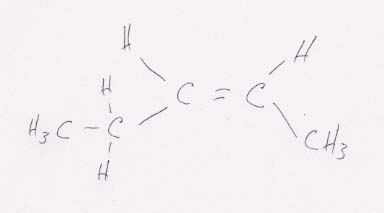
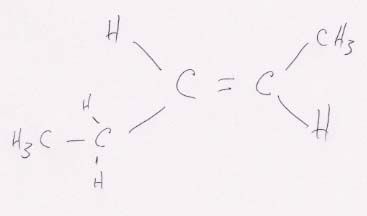
This structure can have cis-trans isomers because the two H's can either be on the same side of the double bond, as here (cis), or diagonally across the double bond from one another, as below (trans).
67. F2+ will have a greater bond energy because it has two fewer antibonding electrons.

71. You construct the diagrams by inserting the proper numbers of electrons in the various levels. CN+ has the same number of outer-shell electrons as C2 does (8), while CN- has the same number as N2 (10). That gives the energy-level diagrams shown below:

(a) Which ion has the stronger
carbon-to-nitrogen bond? CN-, because it has eight electrons in
bonding orbitals rather than the six of CN+.
(b) Is either of these ions paramagnetic? No, because
neither has any unpaired electrons.
75. (a) Structural formula for p-bromotoluene.

The para- means that the Br group will be opposite the CH3 group.
(b) Structural formula for m-diethylbenzene.
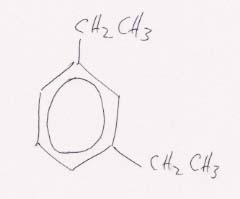
The meta- means that the two ethyl groups will be two positions apart around the benzene ring.
(c) Structural formula for 2,4-diiodotoluene.
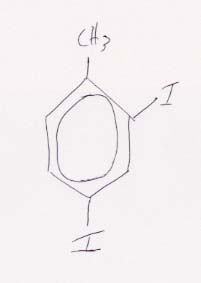
The 2,4- means that the iodine groups will be at positions 2 and 4 from the methyl group in toluene, where the methyl group is defined to be at position 1.
79. The basic Lewis structure for azobenzene is:
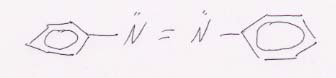
(a) Note that across the double bond there are two unshared pairs of electrons and two benzene rings. This allows cis-trans isomerism between the electrons and the rings:

(b) The C atoms will be bonded with sp2
hybrid orbitals because each C has three electron groups around it. The
delocalized electrons are in π molecular
orbitals. The N atoms are also bonded with sp2 hybrids
(because they have three electron groups) and with a π orbital as part of
the double bonds.
(c) All bond angles are 120° because of their sp2
nature. In the case of the C–N–N bonds, though, they are less than 120°
because the unshared pair of electrons on the N atoms squeezes the angles.
81. The greater the difference in electronegativity between atoms, the greater the dipole moment of their molecule will be. Although this statement is correct for diatomic molecules, it is not necessarily correct for molecules with more than two atoms because the dipoles of the various bonds may partially or completely cancel each other.
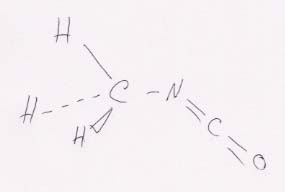
89. Questions on methyl isocyanate, CH3CNO.
(a) Lewis structure:
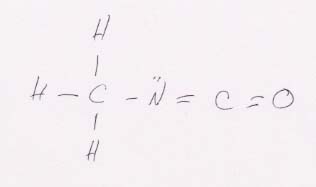
(b) Hybridization scheme for its central atoms. The C of CH3 has sp3 hybrids. The C of NCO has sp hybrids. The N has sp2 hybrids.
(c) Approximate bond angles. The tetrahedral structure on the left has angles of about 109.5°; the CNC angle will be a bit less than 120°, and the NCO angle will be 180°.
(d) Rough sketch of the molecule.
93.
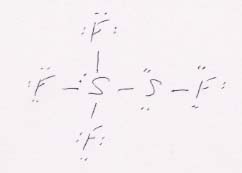
This "horizontal" Lewis structure of this molecule, following directly from its condensed formula in the book, is of a form that is unusual for us. It makes more sense when oriented "vertically," as shown below.

Now we can see a trigonal bipyramidal electronic structure and a seesaw molecular structure for the uppermost five atoms, combined with a bent structure for the lowest three atoms. The upper S atom has sp3d hybrid orbitals, while the lower S atom has sp3 hybrids.