Answers to selected problems, Chapter 6
Review questions
19. Equations for standard enthalpies of formation must always
be written for one mole of the substance in question. Whereas we would usually
write the balanced equation of formation for Fe2O3 as 4
Fe(s) + 3 O2(g) ® 2 Fe2O3(s)
(to avoid a fractional coefficient for O2), for the purposes of
enthalpy we must write it as 2 Fe(s) + 1.5 O2(g) ®
Fe2O3(s).
21. H2(g) gets a standard enthalpy of formation of zero because it is the elemental state of hydrogen. H+(aq) gets a standard enthalpy of formation of zero because it serves as the reference point for other ions (by convention).
Problems
25. Change in internal energy = (energy in) - (energy out) = 455
J - 325 J = 130 J.
31. The reaction is endothermic because it requires energy to
make it go, that is, it absorbs energy. In other words, it is not spontaneous.
q = (134 kJ/10 g H2O)(18
g H2O/mol H2O) = 241 kJ/ mol H2O. This q
is enthalpy, DH, because the problem specifies
that the reaction proceeds at constant pressure rather than at constant volume.
In this sense, it is like most of the reactions in the real world.
35. We start with the reaction 3 Fe2O3(s)
+ CO(g) ® 2 Fe3O4(s) + CO2(g)
DH = -46 kJ
(a) The reaction Fe2O3(s)
+ 1/3 CO(g) ® 2/3 Fe3O4(s) +
1/3 CO2(g) is 1/3 of the initial one, so its change in enthalpy is
1/3 of the first, or -15.3 kJ. (See bottom of page 241 and top of 242.)
(b) The reaction Fe3O4(s)
+ 1/2 CO2(g) ® 3/2 Fe2O3(s)
+ 1/2 CO(g) is 1/2 the opposite of the first one, so its change in enthalpy is
-1/2 of that one's, or 23 kJ.
39. Given the reaction to make calcium hydroxide: CaO(s) + H2O(l) ® Ca(OH)2(s) DH = -65.2 kJ. Remember that the value of DH cited always refers to one "mole" of the reaction, or 56 g of CaO, 18 g of H2O, etc. The problem says that 0.500 kg CaO will be used, which corresponds to (500. g)/(56 g/mol) = 8.93 mol CaO. Thus the change in enthalpy will be (8.93 mol CaO)(-65.2 kJ/mol CaO) = -582 kJ, or 582 kJ given off.
45. The trick here is to recognize that you can only determine
the actual DH of the second equation when you
know how many moles of methane are being oxidized, because the reaction as
written refers to 1 mole of methane. So you use the ideal gas law to solve for n
and adjust the change in enthalpy to this number of moles. Then you apply that
answer to the first equation and find the number of moles of CaO required, again
by scaling. Lastly, convert that number of moles to grams of CaO.
(a) Find the number of moles of CH4
in 1.090 L, 772 Torr, and 21.5 °C. n = PV/RT = [772 Torr/(760 Torr/atm)](1.00
L)/[(0.0821 L atm mol-1 K-1)(273 °C+ 25 °C)] = 0.04152
mol CH4.
(b) Find the actual change in
enthalpy of the second reaction for 0.04152 mol CH4. (-890.3 kJ/mol
CH4)(0.04152 mol CH4) = -36.96 kJ per 0.04152 mol CH4.
(c) Find the number of moles of the
first reaction needed to liberate 36.96 kJ. 1 mole of the reaction is to -65.2
kJ as x moles of the reaction is to -36.96 kJ. This is equivalent to 1/x =
-65.2/-36.96, or x = 0.567 mol reaction.
(d) Find the number of grams of CaO
in 0.567 mol. (0.567 mol CaO)(56 g CaO/mol CaO) = 31.7 g CaO needed. (The
official answer is 32.2 g; the differences come from using exact temperatures
and atomic weights.)
49. The heat capacity of the piece of iron is just the heat required to raise its temperature by 1 °C (from q = CDT). This is 112 J/27 °C = 4.1 J/°C.
57. Solve this problem by equating the heat lost by the iron to
that gained by the water and noting that the final temperatures of iron and
water will be the same. As always, set up the problem algebraically first. CFeDTFe
= CH2ODTH2O. This
becomes (MFe)(Specific heat Fe)(DTFe)
= (MH2O)(Specific heat H2O)(DTH2O).
This in turn becomes (MFe)(Specific heat Fe)(TFe - 39.6
°C) = (MH2O)(Specific heat H2O)(39.6 °C - 23.3 °C). Now
just solve for TFe.
TFe - 39.6 °C = [(MH2O)(Specific heat H2O)(39.6
°C - 23.3 °C)]/[(MFe)(Specific heat Fe)]
= [(817 g)(4.182 J g-1 °C-1)(16.3 °C)]/[(1350 g)(0.449 J
g-1 °C-1)]
= 91.88 °C = TFe - 39.6 °C
TFe = 131.5 °C.
61. Remembering that DH
is written for one mole of reactants (because all coefficients in the equations
are unity), the steps to solve this problem are (1) determine the total heat
given off in the calorimeter, and (2) expressing this per mole of reactants.
(1) The total heat released by the reaction is q cal = (mwater
g)(specific heatwater)(DTwater)
= (1000 mL)(1.000 g mL-1)(4.18 J g-1 °C-1)(23.21
°C - 20.00 °C) = 13,418 J.
(2) Total moles of reactants = (0.5000 L)(0.500 M NaOH) =
0.250 mol NaOH and (0.5000 L)(0.500 M HCl) = 0.250 mol HCl. Thus the heat given
off per mole is 13.418 kJ/0.250 mol = 53.7 kJ mol-1. This makes DH
=
-53.7 kJ mol-1. The final equation for the reaction would thus be written:
HCl(aq) + NaOH(aq) ® NaCl(aq) +
H2O(l) DH
= -53.7 kJ
65. The steps here are: (1) Determine the total energy given off
by the reaction to the calorimeter; (2) Divide by the number of grams of coal
burned.
(1) Total energy given off = heat capacity of calorimeter x
change in temperature of calorimeter = (4.62 kJ °C-1)(22.28 °C -
20.45 °C) = (4.62 kJ °C-1)(1.83 °C) = 8.45 kJ.
(2) -8.45 kJ/0.309 g = -27.4 kJ g-1 coal.
71. To solve this problem, you reverse the second equation and add it to the first:
(Reversed
second) 2 NO2(g)
® N2(g) + 2 O2(g)
DH
= -33.2 kJ
(First)
N2(g) + 2 O2(g) ® N2O4(g)
DH
= +9.2 kJ
(Sum)
2 NO2(g)
® N2O4(g)
DH
= -24.0 kJ
Note how according to Hess's law, reversing the equation changes the sign of DH.
75. This problem is just like the previous one, except with one more equation. So that you can follow my logic, here are the three starting equations and the final equation:
Starting:
N2(g) + 3 H2(g) ®
2 NH3(g)
N2(g) + O2(g) ®
2 NO(g)
2 H2(g) + O2(g) ®
2 H2O(l)
Final:
4 NH3(g) + 5 O2(g) ®
4 NO(g) + 6 H2O(l)
The approach here is the same as with
balancing equations: find terms in the final equation that appear in only one of
the starting equations and see how the starting equation needs to be multiplied
to match the final equation. If that approach doesn't cover all the starting
equations, work with the others first and look to the last step to see what
multiple of the other starting equations is needed.
The first equation is the only one with NH3.
Because the final equation contains twice any many NH3's as the
first, and on the other side, multiply the first equation by -2.
The second equation is the only one with NO. The final
equation contains twice as many NO's as the first, and on the same side.
Therefore, multiple the first equation by 2.
(You confirm that the second equation must be handled
oppositely to the first because both contain 1 N2 on their left
sides, which must cancel because N2 appears in neither the third equation nor
the final one.)
The third equation is the only starting equation to contain H2O.
The final equation contains three times as many H2O's, and on the
same side. Therefore, multiply the third equation by 3.
Here are the multiplied starting equations and the final equation, with terms cancelled out as appropriate:
4 NH3(g)
® 2 N2(g) + 6 H2(g)
2 N2(g) + 2 O2(g) ®
4 NO(g)
6 H2(g) + 3 O2(g) ®
6 H2O(l)
4 NH3(g) + 5 O2(g) ®
4 NO(g) + 6 H2O(l)
Treating the changes in enthalpy with the same coefficients gives -2(-92.22 kJ) + 2(180.5 kJ) + 3(-571.6 kJ) = -1169.4 kJ = -1169 kJ.
77. To calculate the standard enthalpy change for each reaction,
just add the standard values for the substances of the left and subtract those
on the right. (See pages 256 ff. in the text.) Of course, some of the values may
in general have to be multiplied by small integer factors that are needed to
create the final reaction from its component reactions (as shown in the previous
problem), but that is not needed here because of the simple reactions involved.
(Problem 78 needs this approach, however.)
(a) DH° = DHf°
[NH4Cl(s)] - DHf° [NH3(g)]
- DHf° [HCl(g)] = (-314.4) -
(-46.11) - (-92.31) = -176.0 kJ
(b), (c), and (d) are done similarly.
83. As in problem 77, DH° = DHf° [NH3(g)] + DHf° [H2O(l)] - DHf° [NH3+(aq)] - DHf° [OH-(aq)] = 46.11 kJ + (-285.8 kJ) - (-132.5 kJ) - (-230.0 kJ) = 30.6 kJ.
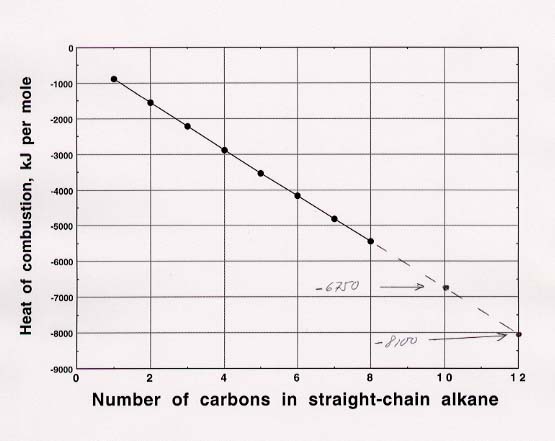
85. (a) I have prepared two plots, one for the heats of combustion per mole of compound and the other for heats of combustion per mole of C (what the problem actually requested).

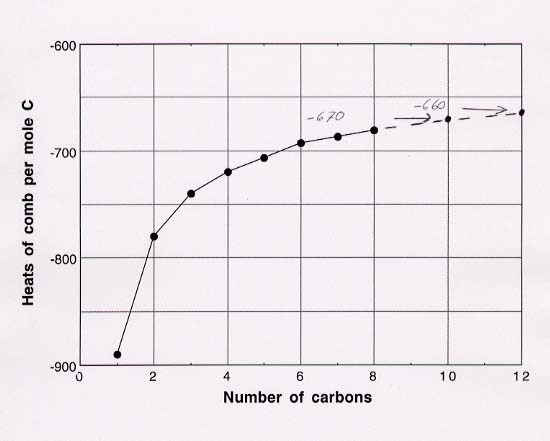
(b) Estimates of heats of combustion can be read
directly from the first plot: -6750 kJ per mole for decane and -8100 kJ per mole
for dodecane. Estimates from second plot, which will be more reliable because
they essentially deal with differences from one point to the next, are obtained
by multiplying the heat per mole of carbon by the number of moles of carbon:
-670 kJ x 10 moles carbon per mole of substance = -6700 kJ per mole for decane
and -660 x 12 moles per mole of substance = -7900 for dodecane. It is
possible to get results that are slightly more accurate by extrapolating
mathematically rather than by reading directly from the plots, but we won't
bother with that here.
91. (a) That his bride was watching in wonderment, realizing for
the first time what a total nerd she had married!
(b) Seriously, Joule could have foreseen two
effects on the temperature of the falling water: a slight warming when the water
hit bottom, as its kinetic energy is degraded to heat, and a slight cooling of
the water by evaporation as it was falling. These two small effects would have
made smaller to the extent that they cancelled one another. With Joule's bride
looking over his shoulder, he probably was in no position to observe small
effects like these, and he would have observed no change in temperature. This of
course would have angered his bride still more, and she might have said
something like, "James, dear, if you are going to leave me on our honeymoon
for some dumb temperature under a waterfall, you can at least find some sort of
change!" (His bride didn't appreciate that no change is just as significant
a result as a change.)
(c) There is, of course, a third
possibility, one that all PC folks will appreciate, namely that Joule married a
scientist and that she encouraged him to check the temperature at the
bottom of the waterfall. Maybe it was even her idea!
97. The basic equation is CH4(g) + 2 O2(g)
® CO2(g) + 2 H2O DH
= -890.3 kJ.
(a) To determine how much mass of CH4(g)
must be burned to give off 1.00x105 kJ, you divide this number by
890.3 kJ per mole of CH4(g), and get 112.3 moles of CH4.
You then multiply this by the molecular mass of CH4, 16 g/mol, and
get 1797 g of CH4.
(b) For this part, you must first find how many moles of CH4
are contained in 105 L at 23 °C and 746 mmHg. That is a simple application of
the ideal gas law: n = PV/RT = (746 mmHg/760 mmHg atm-1)(105
L)/[(0.0821 L atm mol-1 K-1)(298 K)] = 4.21 mol CH4.
Next, scale the equation to this number of moles:
(1 mol CH4/4.21 mol CH4)/(890.3 kJ/x
kJ) ® x = (4.21)(890.3) = 3750 kJ.
103.