Answers to selected problems, Chapter 8
Note: 1 the up and down arrows needed in several of these
questions could not be made to reproduce in both Internet Explorer and Netscape.
I chose Internet Explorer 5 because that is my default browser. If you don't
have access to IE 5, I will give you the printed version of these answers.
Note 2: For tutorial purposes, I have generally written the
electron configurations in the order in which the subshells are filled. This
practice differs from that of the book, which generally lists them by
"position" instead.
Review questions
3. The principle at work in this question is that for the
hydrogen atom, the energy levels of orbitals within a principal shell are all
the same, whereas for atoms of other elements, the energy levels of all orbitals
are different. (See point 1 in the middle of page 317 and Figure 8.1 on the same
page.)
(a) The three orbitals listed for this part (1s, 2s,
2p), are at different energy levels for atoms of any element because they
come from different shells. Orbitals 2s and 2p are at identical levels for
hydrogen, though.
(b) The three orbitals listed here (3s, 3p, 3d)
will be at the same energy levels for hydrogen but not for other elements.
(c) The three orbitals listed here (3px, 3py,
3px) will be at the same energy levels for hydrogen and for
other elements.
10. What subshell(s) is(are) being filled in these regions of
the periodic table?
(a) Groups 1A and 2A--the various s subshells 1s,
2s, etc.
(b) Groups 3A through 7A--the p subshells 2p, 3p,
etc.
(c) the transition elements--the d subshells 3d,
4d, etc., but where 3d is being filled in row 4, 4d
in row 5, etc.
(d) the lanthanides and actinides--the f subshells 4f
and 5f, but where 4f is being filled in row 6, etc.
18. Must all atoms with odd atomic numbers be paramagnetic?
Yes. All paramagnetic elements have at least one odd electron.
Must all atoms with even atomic numbers be diamagnetic?
No, because an element with even atomic number may have two, four, etc. single
electrons in different shells and thus be paramagnetic. An example is carbon: 1s22s22px12py1.
Problems
29. Which orbital diagrams are possible and which not?
(a) Not possible because one of the 2p pairs of
electrons have the same (positive) spins.
(b) Not possible because the last orbital contains three
electrons (two is the maximum per orbital).
(c) Possible--the orbital diagram of nitrogen.
(d) Allowed, but the spins are by convention drawn upward
first.
(e) Not possible because the first three (unpaired) electrons
in the 2p subshell must all have the same spin.
(f) Possible--the orbital diagram of magnesium.
33. Explain why each of these electron configurations is
wrong.
(a) 1s22s63s2--The
2s orbital can have only two electrons, not the six indicated here.
(b) 1s22s22p73s1--The
2p subshell can contain only six electrons, not the seven shown here.
(c) 1s22s22p62d3--There
is no 2d subshell.
35. Use the spdf notation and the Aufbau principle
(Figure 8.2, page 320) to write the electronic configuration for each of these
elements:
(a) Al (Z = 13) 1s22s22p63s23p1
(b) Cl (Z = 17) 1s22s22p63s23p5
(c) Na (Z = 11) 1s22s22p63s1
(d) B (Z = 5) 1s22s22p4
(e) He (Z = 2) 1s2
(f) O (Z = 8) 1s22s22p4
(g) C (Z = 6) 1s22s22p2
(h) Li (Z = 3) 1s22s1
(i) Si (Z = 14) 1s22s22p63s23p2
39. Orbital diagrams for the ground-state electronic
configurations for atoms of the following elements.
(a) C (Z = 6) 1s
↑↓ 2s
↑↓ 2p
↑ ↑
(b) O (Z = 8) 1s ↑↓
2s ↑↓
2p ↑↓↑ ↑
(c) K (Z = 19) 1s ↑↓
2s ↑↓
2p ↑↓↑↓↑↓
3s ↑↓ 2p
↑↓↑↓↑↓ 4s
↑
(d) Al (Z = 13) 1s ↑↓
2s ↑↓ 2p
↑↓↑↓↑↓ 3s
↑↓ 2p ↑
(e) S (Z = 16) 1s ↑↓
2s ↑↓
2p ↑↓↑↓↑↓
3s ↑↓ 2p ↑↓↑ ↑
(f) Mg (Z = 12) 1s ↑↓
2s
↑↓ 2p ↑↓↑↓↑↓
3s ↑↓
43. Orbital diagrams for these ions.
(a) Br- The short answer is [Kr], because
Br's extra electron gives it the same electronic configuration as Kr has.
The long answer is [Ar] 3d
↑↓ ↑↓ ↑↓
↑↓ ↑↓ 4s ↑↓
4p ↑↓↑↓↑↓.
Be careful, though, because the electronic configurations, in the form that reproduces the
order in which the subshells are filled (see discussion on page 324 of the two
styles of writing electron configurations), would be [Ar] 4s23d104p6.
It may be easier for you to start with the electronic configuration, for which
you can use the Aufbau diagram in Figure 8.2, and then convert the order of the
orbitals to that required for the orbital diagrams, which go by
"position" rather than by energy.
(b) Ni2+ Careful! The electronic
configuration of Ni is [Ar] 4s23d8, which
reflects the sequence of adding the electrons after the argon core. In terms of
"position," they are written in the orbital diagram as [Ar] 3d ↑↓↑↓↑↓↑
↑ 4s
↑↓ . (Note the blank spaces after the
last two up-arrows in 3d.) The two electrons stripped to create Ni2+
come from the outermost shell, or 4s2. Thus, Ni2+
is [Ar] 3d8, or [Ar] 3d ↑↓↑↓↑↓↑
↑ . Whew!
(c) Sb3+ The configuration of
regular Sb (in sequence) is [Kr] 5s24d105p3.
Giving it a triply positive charge means removing the last three electrons,
which will give [Kr] 5s24d10. The orbital
diagram for this is [Kr] 4d ↑↓
↑↓ ↑↓ ↑↓ ↑↓ 5s
↑↓ .
(d) Te2- The configuration for Te
is like that of Sb but with one more electron: [Kr] 5s24d105p4.
Adding two electrons fills the 5p subshell, for [Kr] 4d105s25p6
(by position). That gives [Xe], or [Kr]
4d ↑↓↑↓↑↓↑↓↑↓
5s ↑↓
5p ↑↓↑↓↑↓
.
47. (a) Bi has five valence electrons (6s26p3).
The 5d and 4f electrons in between are not in the valence shell
(6).
(b) The electrons in the fourth principal shell of Au are 4s24p64d104f14
= 32 in number.
(c) Elements with five valence-shell electrons are those in
Group 5A, or N, P, As, Sb, Bi.
(d) Any unpaired electron in Se will be found in its valence
shell, whose electrons are 4s24p4. By Hund's
rule, the four electrons will enter the 4p subshell as follows: 4px4py4pz4px,
which will leave 4py and 4pz unpaired.
(e) There are ten transition elements in the fifth period: Y,
Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, and Cd. Note that these transition elements are
in the fifth period but their transition electrons are 4d10.
49. To determine whether a given species is diamagnetic or
paramagnetic, examine its orbital structure for unpaired electrons. If any are
present, the species is paramagnetic. If not, it is diamagnetic.
(a) S has two unpaired electrons, and so is paramagnetic: [Ne]
3s23px23py3pz.
(b) Ba has no unpaired electrons, and so in diamagnetic: [Xe]
6s2.
(c) Careful! V2+ has three unpaired electrons, and
so is paramagnetic. This problem is like 43b. The electronic structure of V is [Ar]
4s23d3. The two electrons that are stripped
to make the ion come from the outer shell, however, which leaves [Ar] 3d3.
The 3d electrons enter in the sequence 3dxy3dxz3dyz,
all unpaired (Hund's rule).
(d) O2- has the electronic configuration of [Ne],
in which all electrons are paired: 1s22s22p6
. It is thus diamagnetic.
(e) Ag's electronic configuration is [Kr] 5s24d9.
Ag is one of those transition elements whose electronic structure is
exceptional. The nine 4d electrons should enter as 4dxy24dxz24dyz24dx2-y224dz,
but it "borrows" one electron from the 5s2 to fill
the 4d subshell and give 5s14d10 (see
page 326). One electron remains unpaired, though, as it must.
Comment: Of course, one easy way to approach this problem is
to recognize that any element with an odd atomic number must have at least one
unpaired electron, and so will be paramagnetic. (The converse in not necessarily
true, however, because even-numbered atoms can easily have two or four unpaired
electrons--see S above.) S is even but with two unpaired electrons, Ba is even
with no unpaired electrons, V2+ is odd minus two, or still odd, O2- is even plus
two makes full shell, and Ag is odd.
53. Arranging atoms in order of increasing atomic
radius:
(a) Al, Mg, and Na are all in the third A-row of the periodic
table. Since atomic radii of atoms decrease to the right along any A-row, the
sequence of radii will be Al < Mg < Na.
(b) Ca, Mg, and Sr are all in the second group. Atomic radii
increase down a group. This gives the sequence of radii Mg < Ca < Sr.
57. (a) You can confidently predict that Ca is larger than
Cl because or two factors acting in the same direction: moving leftward from Cl
to Mg will increase the radii of the atoms, and moving downward from Mg to Ca
will also increase radii.
(b) Ill-defined; can't answer reliably. There are four steps
to reasoning out this answer.
(1) K+ is [Ar] but with
one more proton in the nucleus, so that K+ < [Ar].
(2) F- is [Ne] but with one fewer
proton in the nucleus, so that F- > [Ne].
(3) [Ne] < [Ar].
(4) So in comparing the sizes of K+
and F-, we are comparing < [Ar] with > [Ne], or < [Ar] with > (<
[Ar]). Without knowing the magnitudes of the < and > factors, this can't
be done.
59. (a) Because ionization energies decrease down the
periodic table, Ba < Ca < Mg.
(b) Because ionization energies decrease with increasing size
and size increases leftward across the periodic table, ionization energies will
increase rightward across the table. Thus, Al < P < Cl.
(c) This problem just involves a lot of bookkeeping. Ne is a
noble gas with a high first ionization energy. F is above Cl, so it will have an
greater ionization energy. Cl is to the right of Na, so Cl will have a higher
ionization energy. Fe, a transition metal, has about the same ionization energy
as Ca, which is great than Na (Table 8.4) but less than Cl (deduction fro same
table). Thus the order of ionization energies is Na < Fe < Cl < F <
Ne.
65. Silicon has a greater electron affinity than P does because Si, with only 2 3p electrons, has one empty 3p orbital (3pz) where the additional electron can go without interacting strongly with any existing 3p electrons. P, with one additional electron, has one in each of its 3p orbitals, so the next electron would have to enter an orbital that already contains one electron. Since there is less "resistance" to putting an electron into an empty orbital than into one that is half full, Si will have the greater electron affinity.
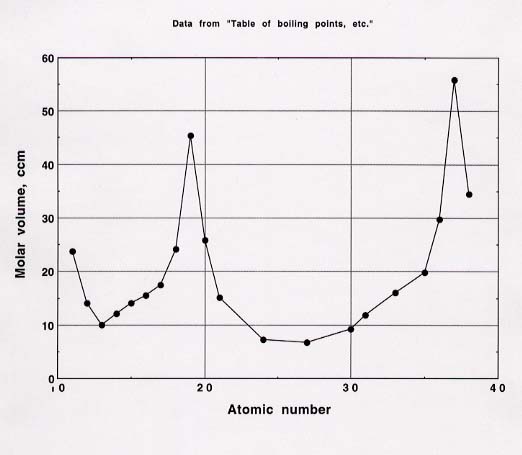
67. The graph of atomic volume (as volume per mole of atoms) is shown below. This curve resembles the curve for atomic radius versus atomic number, which is Figure 8.9 in the text.

71. This is an easy problem. Tl is lowest and farthest to the left, so it will have the lowest ionization energy. Ne, highest and farthest to the right, will have the highest. From Tl, next-lowest is Ge, above and to the right. Then comes Se, to its right, followed by S, which is directly above Se. From there, Ne is above and to the right, so it has higher ionization energy still. Thus the sequence of increasing ionization energies is Tl < Ge < Se < S < Ne.
75. (a) Completing the equation: N2O5(s)
+ H2O(l) ® HNO3(aq)
Balancing the
equation: N2O5(s) + H2O(l) ®
2 HNO3(aq)
(b) Completing the equation: MgO(s) + CH3COOH(aq) ®
Mg(CH3COO)2(aq) + H2O(l)
Balancing the
equation: MgO(s) + 2 CH3COOH(aq) ®
Mg(CH3COO)2(aq) + H2O(l)
(c) Completing the equation: Li2O(s) + H2O(l)
® LiOH(aq)
Balancing the
equation: Li2O(s) + H2O(l) ®
2 LiOH(aq)
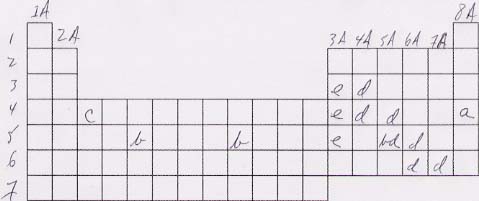
77. The answers are shown in the periodic table below.
81.
83.
89.