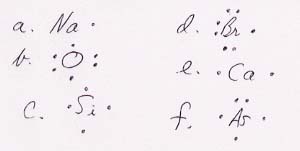
Answers to selected problems, Chapter 9
Review questions
6. Lewis symbols.
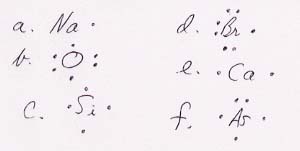
12.

16. (a) F is more electronegative than Br because F is smaller
(higher in the periodic table).
(b) Br is more electronegative than Se because it is more to
the right in the table.
(c) Cl is more electronegative than As because it is more
toward the upper right in the periodic table.
(d) N is more electronegative than H (I don't think there is
any obvious way to get this from the periodic table alone because N is below H,
which would give it a lower electronegativity, but is farther to the right,
which would give it a higher electronegativity).
25. Unsaturated hydrocarbons are compounds that contain only carbon and hydrogen and have double or triple bonds.

Problems
29. Which of these ions have noble-gas electronic
configurations?
(a) Cr3+ 1s22s22p63s23p63d3
Not noble gas (4s electrons stripped first)
(b) Sc3+ 1s22s22p63s23p6
Noble gas
(c) Zn2+ 1s22s22p63s23p63d10
Not noble gas
(d) Te2- 1s22s22p63s23p63d104s24p64d105s25p6
Noble gas
(e) Zr4+ 1s22s22p63s23p63d104s24p6
Noble gas
(f) Cu+ 1s22s22p63s23p63d10
Not noble gas (N.B. same as Zn2+)
31.
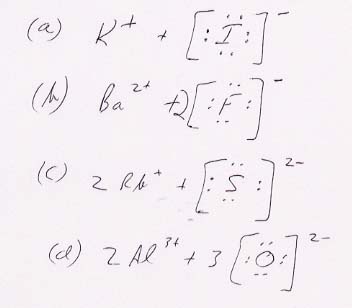
35.
39.

43. (a) B < N < F (Electronegativities increase left to
right across
rows in the periodic table.)
(b) Ca < As < Br (Same reason as a)
(c) Ga < C < O (Increasing up and to the right in the
periodic table)
49. (a) C normally has four outer electrons. Here it has 4
unshared and 4 shared, which makes 4 + 0.5(4) = 6. That gives it a formal charge
of -2.
N normally
has five outer electrons. Here it is has 8 shared, or 0.5(8) = 4. Thus its formal
charge is +1.
O normally
has six outer electrons. Here is has 4 unshared plus 4 shared, or 4 + 0.5(4) =
6. Thus its formal charge is 0.
The total
formal charge is -2 + 1 = -1, the same as the charge on the ion. All is OK.
(b) Done the same way as in (a). The charge on N = 5 - [2 +
0.5(6)] = 0. The charge on C = 4 - [0.5(8)] = 0. The charge on O = 6 - [6 + 0.5(2)] =
-1. Total formal charge is 0 + 0 + (-1) = -1, the same as on the ion.
(c) Which structure is preferred? Structure b, because it has less
charge on the atoms.
51. To write structures for the bicarbonate ion, place the C in the center and bond it to two O's and one OH, as the formula HOCO2- suggests, and give the ion a single negative charge. The resulting structures are:
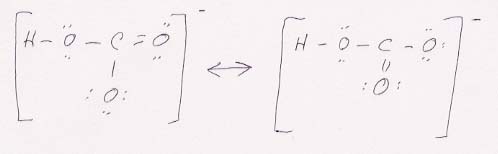
The OH takes one of the carbon's four bonds. The other three bonds to carbon are to be divided between the two O's, a single bond to one and a double bond to the other. The two resonance structures differ in which O gets which bond. Together, they are equivalent to each O getting 1.5 bonds.
55. The Lewis structures are:
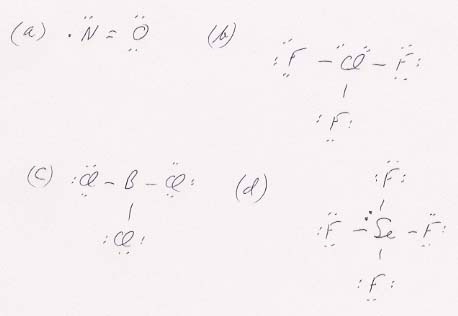
Comments: (a) NO has an odd electron. (b) The Cl in ClF3 has an expanded shell (10 electrons). (c) The B in BCl3 has an incomplete outer shell (6 electrons). (d) The Se in SeF4 has an expanded outer shell.
61. The figure shows the wrong structures first and the correct structures second.

(a) was wrong because it maldistributed the electrons between S
and C, giving C too few and S and C improper formal charges.
(b) was wrong because it had one electron too few. Adding the electron requires
the two resonance structures shown.
(c) was wrong because it maldistributed the electrons between the N and the
right-hand Cl.
65. (a) The bond length for I—Cl
is the average of the bond lengths for I—I
and Cl—Cl, or 0.5(266 pm + 199 pm) = 233
pm.
(b) The same procedure for C—F
gives 0.5(154 pm + 143 pm) = 149 pm.
69. The bond length for nitrogen to nitrogen (123 pm) means that is a double bond (Table 9.1). The bond between nitrogen and fluorine can only be single; as confirmation, its length (141 pm) is also consistent with averaging F—F and N—N, not F—F and N==N. The structure is then:
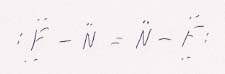
75. In terms of bonds, this reaction may be written as:

The change in enthalpy for this reaction is the change in enthalpy for the bonds broken minus that for the bonds formed. If you imagine that there is only a single oxygen-oxygen bond energy in ozone, you can envision this process as breaking both those bonds (by destroying the ozone) and creating two double bonds in the oxygen molecules (that are formed). In equation form:
ΔHrxn = ΔHbroken
bonds + ΔHformed bonds
-391.9 kJ = 2ΔHO3 + 2ΔHO2
-391.9 kJ = 2ΔHO3 + 2 mol (-498 kJ mol-1)
ΔHO3 = 302 kJ/O3 bond
79. (a) Ethene is a two-carbon "chain" with a
double bond: H2C=CH2
(b)1-butene is a four-carbon chain
with the double bond at the first carbon: H2C=CHCH2CH3
(c) Propyne is a three-carbon chain
with a triple bond: HC≡CCH3
(d) 2-pentyne is a five-carbon chain
with a triple bond at the third carbon: H3CC≡CCH2CH3
83. The reaction is:
C2H4(g) + H2(g) → C2H6(g) ΔH = ??
(a) Find ΔH by using average bond energies. As above, the enthalpy of reaction equals that of bonds broken plus bonds formed. Imagine that every bond in the reactants is broken and every bond in the products is formed. That gives 4 C–H + 1 C=C + 1 H–H broken and 6 C–H and 1 C–C formed. In terms of ΔH:
ΔH = 4ΔHC–H + 1ΔHC=C + 1ΔHH–H - 6ΔHC–H - 1ΔHC–C = 4(414 kJ) + 1(611 kJ) + 1(436) - 6(414 kJ) -1(347 kJ) = -128 kJ mol-1.
(b) Find ΔH by using enthalpies of formation. The change in enthalpy for the reaction is just the enthalpy of formation of the products (which are formed) minus the enthalpies of formation of the reactants (which are destroyed). That becomes:
ΔHrxn = ΔHf (C2H6) - ΔHf (C2H4) - ΔHf (H2) = (from Appendix C) -84.68 kJ mol-1 - (52.26 kJ mol-1) - (0) = -136.94 kJ mol-1.
(c) The result from enthalpies of formation is more negative than that from bond energies, but they are close.
87. The Lewis structures of alkanes are the same as their structural formulas because saturated hydrocarbons do not have unpaired electrons that could lead to resonance structures. Some other organic compounds have single electrons, however.
93. The short bond lengths for the two N-N bonds mean that they cannot be single bonds. They are consistent with one double bond and one triple bond. Thus the Lewis structure will be

97.
101.