Answers to Assignment , Chapter 7
OCG123, Spring 2001
Review Questions
1. The residence time of carbon is shortest in plants and the oceans, the
figures being 600 Gt/60 Gt y-1 = 10 years for plants and 37,000/0.67
= 55,000 years for bicarbonate in the oceans. The value for sedimentary
limestone is 40,000,000/0.20 = 200 million years.
2. The precipitation of calcite skeletons is strictly inorganic (involving CO32-). The exchange of carbon between oceans and atmosphere involves CO2 dissolving in the seawater, and so is inorganic. Dissolution from the sea floor certainly involves organic materials deposited there, as does oxidation of organic materials during weathering.
3. The biological pump is the falling of particles of organic matter such as dead phytoplankton, dead zooplankton, and fecal pellets from the surface ocean into the deep ocean and the release of nutrients at these lower levels by decomposition. (See discussion on page 136.)
4. Plate tectonics maintains carbon in the oceans and atmospheres by returning it from subducted continents to oceans and atmosphere by volcanic action. (See Figure 7-17 and associated discussion.)
5. Weathering of carbonate rock does not lead to net removal of CO2 from the atmosphere because the bicarbonate ion created as H2CO3 weathers the limestone is reconverted to H2CO3 as CaCO3 is precipitated from the oceans to the seafloor. The net result of the weathering and precipitation reactions (their sum) is to transfer limestone (CaCO3) from terrestrial sediment to marine sediment, with CO2 (or H2CO3) acting as a catalyst. (See the equations on p. 146.)
Critical-Thinking Problems
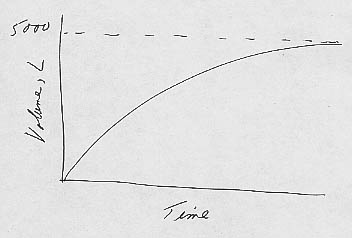
1. a. k = outflow rate/volume of reservoir = 25 lpm/5000 l = 0.005 min-1.
The residence time is the inverse, or 200 min. So k and the residence
time are the inverse of each other.
b. (Words) The size of the initially empty reservoir
increases nearly linearly (25 lpm) as the first water is added to it. The larger
the reservoir gets, however, the faster water can flow out of it. Eventually the
reservoir reaches its steady-state volume of 5000 liters.

4. a. The mean molecular weight of air is just the volume-weighted average of the four constituents:
Mean MW = 0.78(28.0134) + 0.21(32.000) + 0.01(39.948) +
0.00036(44)
= 28.98 = 29.0 to three sig. figs.
b. The number of moles of air is the mass of the
atmosphere divided by its molecular weight, or (5x1021 g)/(29.0
g/mol) = 1.72x1020 moles.
The number of moles of oxygen is the
moles of air multiplied by the volume fraction (= molar fraction) of oxygen:
Moles of O2 = 0.21x(1.72x1020) = 3.62x1019
The number of moles of CO2 is calculated analogously: 0.00036(1.72x1020) = 6.2x1016.
c. We can address this problem by recognizing that burning
one mole of C consumes one more of O2 and produces one mole of CO2. So burning 600 Gt of C would
consume (600x1015 g C)/(12 g mol-1 C) = 5.0x1016
moles of C and therefore make the same number of moles of CO2.
Relative to the original 6.2x1016 moles of CO2 in the
atmosphere, that would represent an 81% increase (5.0/6.2).
The 5.0x1016 moles of O2
consumed in the process would represent a 0.14% decrease in the atmospheric
supply (5.0x1016/3.0x1019).